Introduction: Endemic Burkitt lymphoma (eBL) is a fast-growing B-cell malignancy strongly linked to Epstein-Barr virus (EBV) infection, a member of the herpes virus family. The virus is widespread, infecting 95% of the global population, and has also been linked to several other cancers, including nasopharyngeal carcinoma (NPC), diffuse large B-cell lymphoma (DLBCL), Hodgkin lymphoma (HL), natural killer T-cell lymphoma (NKTCL), gastric carcinoma (GC) and leiomyosarcomas. The distribution of these cancers is inconsistent in different regions, raising the hypothesis of whether different strains are linked to specific cancers. Using high throughput sequencing, we obtained EBV whole genome sequences from the plasma of children with eBL diagnosis from a large cohort study in East Africa.
Methods: Twenty-one EBV-positive patients (Sixteen BL, one diffuse large B-cell lymphoma, one lymphoblastoid lymphoma, one clear cell sarcoma, one small blue cell tumour and one alveolar rhabdomyosarcoma) were recruited from St Mary's Hospital Lacor, in Uganda, between 2020 and 2023. Venous blood samples were collected in appropriate DNA tubes, and ~ 5 ml of plasma was used for cfDNA extraction using QIAamp circulating Nucleic acid kit (Qiagen, USA). The cfDNA libraries were prepared using the Thruplex Tag- Seq kit (Takara Bio Inc. Japan), target capture and enrichment was performed using an EBV-custom panel (IDT Inc. USA) before sequencing. The Illumina fastq files were processed using an in-house pipeline, and paired-end reads were aligned to a bespoke hybrid EBV reference genome (NC_007605.1 and EBNA genes from NC_009334) using BWA with an alt-ware method. The average sequencing depth and EBV genome coverage were 5164 and 97.8%, respectively. Following the GATK best practice workflows (version 4.0), 15,318 variants were called across all samples using Varscan and Vardict after base and variant recalibration. Functional annotation was performed using the SNPEff package (v5.1d) according to the reference genome (NC_007605.1, NCBI annotation, Nov 2013). For the phylogenetic analysis, 322 public EBV genomes were downloaded from the GenBank and aligned with 21 EBV genomes from the current study using a multiple sequence alignment program (MAFFT v7.52), and the maximum likelihood of the phylogenetic relationship was inferred using RAxML (v8.0), assuming a general time reversible (GTR) model. The inferred phylogeny was subsequently rooted using the evolutionary placement (EPA) algorithm from RAxML using a Macacine herpesvirus 4 genome sequence (NC_006146) as an outer group. The tree was annotated using FigTree software (v1.4.4).
The study was approved by the Oxford Tropical Research Ethics Committee (OxTREC Ref: 15-19), National Institute of Medical Research (NIMR/HQ/R.8a/Vol.IX/3408), Uganda National Council of Science and Technology (Ref: HS529ES), and St Mary's Hospital Lacor Institutional Research Ethics Committee. Participants provided written informed consent and assent for minors.
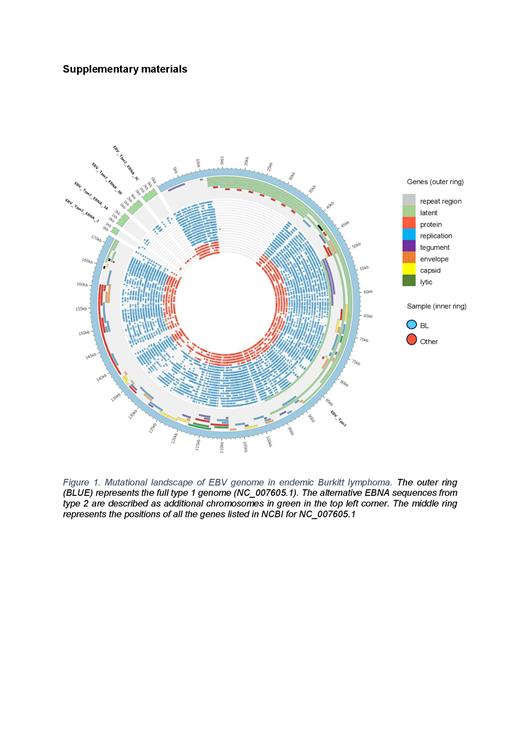
Results: We examined the genomes of 21 EBV samples, including 18 type-1 and 3 type-2 strains. Our analysis revealed an average of 729 mutations per sample, comprised of 707 SNVs and 26 indels (<50bp) per sample (Figure 1). Within 16 eBL tumours, we observed the 30-bp deletion in LMP-1 in 43.8% (7/16) cases. This deletion has been previously reported in NPC in Southeast Asia and is associated with high oncogenic potential and weak immunogenicity. We also identified a novel frameshift variant (15-bp deletion) in LMP-1 in 66.7% (14/21) of tumour samples. The LMP-1 gene encodes a 356-amino acid polypeptide with a cytoplasmic amino terminus, a transmembrane domain, and a cytoplasmic carboxy terminus. The C-terminus interacts with cellular proteins, activating the transcriptional nuclear factor-kB (NF-kB) pathway associated with antiapoptotic cell signalling. Additionally, we observed a high degree of polymorphism in EBNA-1, EBNA3s, LMP-2A, BALF1, and BOLF-1 viral genes. Our phylogenetic analysis revealed distinct clustering of African EBV genomes compared to Asian strains and NPC versus eBL (Figure 2).
Conclusions: Overall, our study provides the first-ever comprehensive mutational profile of EBV in eBL clinical samples, offering valuable insights into the somatic events of EBV in tumour biology.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal